PARADIM Highlight #111—External User Project (2025)
D. Kwabena Bediako (UC Berkeley)
The interplay between symmetry and topology in Magnetic materials enables the design of exotic phases and technologically useful properties. A team from UC Berkeley showed that V1/3NbS2 can crystallize in two distinct superlattices defined by the stacking periodicity of magnetic intercalants. One structure is metallic and exhibits key signatures of altermagnetism, while the other superlattice, which has not been isolated before in this family of materials, is a semimetallic noncollinear antiferromagnet with potential topologically nontrivial properties.
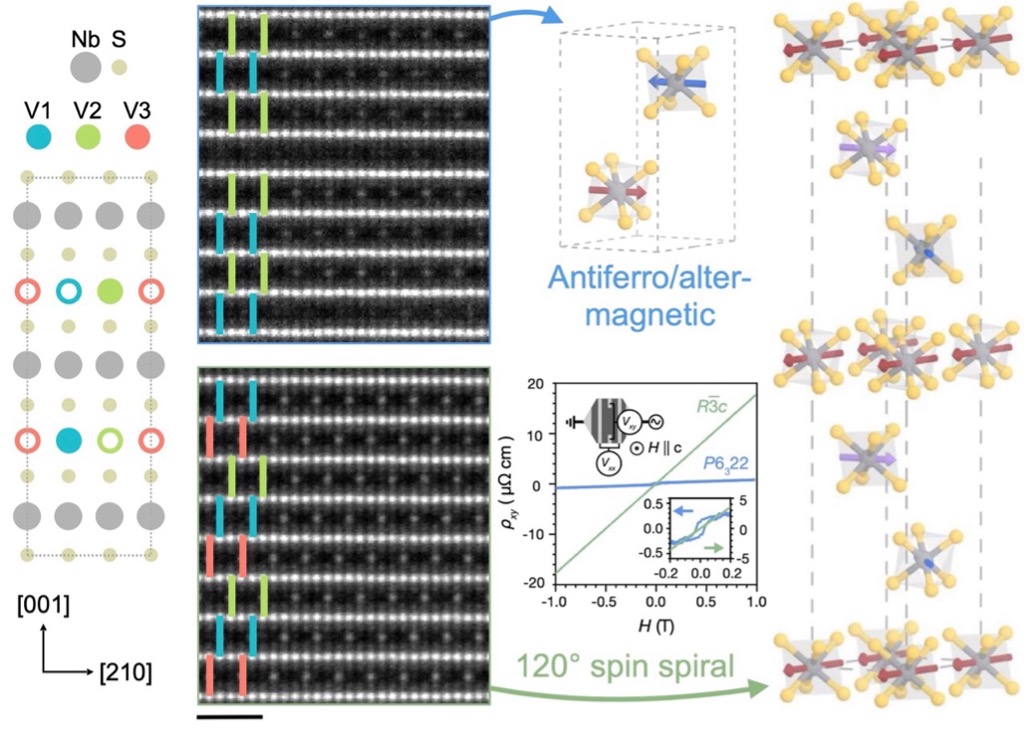
Figure 1. Different vanadium ion intercalant ordering in NbS2, characterized by ABAB (top) and ABC (bottom) stacking, identified by color overlays (scale bar 1 nm). The two crystal structures exhibit distinct magnetic and electronic properties.
This discovery provides a route to systematically engineer the magnetic and electronic behaviors of the broader intercalated transition metal dichalcogenide family, advancing their prospects for low-power spintronic and topological quantum devices. Moreover, the possibility of reversible switching between these intercalant orders via external stimuli suggests opportunities for functional phase-change devices that exploit tunable magnetic and topological states.
This work was made possible by the low-dose imaging and spectroscopy in PARADIM’s high-brightness Spectra “Kraken” STEM required to achieve atomic resolution visualization and elemental mapping of the sensitive V intercalants which are rapidly damaged under more conventional experimental conditions.
The interplay between symmetry and topology in magnetic materials makes it possible to engineer exotic phases and technologically useful properties. A key requirement for these pursuits is achieving control over local crystallographic and magnetic structure, usually through sample morphology (such as synthesis of bulk crystals versus thin films) and application of magnetic or electric fields. Here we show that V1/3NbS2 can be crystallized in two ordered superlattices, distinguished by the periodicity of out-of-plane magnetic intercalants. Whereas one of these structures is metallic and displays the hallmarks of altermagnetism, the other superlattice, which has not been isolated before in this family of intercalation compounds, is a semimetallic noncollinear antiferromagnet that may enable access to topologically nontrivial properties. This observation of an unconventional superlattice structure establishes a powerful route for tailoring the tremendous array of magnetic and electronic behaviors hosted in related materials and may expand their use in low-power spintronic or topological quantum devices.
We reveal a new superlattice structure which has not been previously reported in intercalated transition metal dichalcogenide systems, and demonstrate how different intercalant orderings can give rise to distinct magnetic properties. These results also highlight the importance of comprehensive and complementary characterization techniques in new materials to reliably identify the relevant crystal structures.
This work was made possible by the low-dose imaging and spectroscopy in PARADIM’s high-brightness Spectra “Kraken” STEM required to achieve atomic resolution visualization and elemental mapping of the sensitive V intercalants which are rapidly damaged under more conventional experimental conditions.
The work was initiated by Prof. Bediako (UC Berkeley) via a PARADIM User Proposal.
S.S. Fender, N. Schnitzer, W. Fang, L. Bhatt, D. Huang, A. Malik, O. Gonzalez, V. Sunko, L.S. Xie, D.A. Muller, J. Orenstein, Y. Ping, B.H. Goodge, and D.K. Bediako, "Unconventional Superlattice Ordering in Intercalated Transition Metal Dichalcogenide V1/3NbS2," J. Am. Chem. Soc. 147, 32315-32320 (2025).
We are grateful to M. Frontzek for helpful discussions. We extend our thanks to J. Vigil and P. G. Hegel for assistance with PXRD measurements, as well as H. Jayakumar and J. Liang for assistance with XPS measurements. This material is based upon work supported by the U.S. National Science Foundation, under award no. 2426144 (DKB). Y.P. acknowledges support from the NSF through the University of Wisconsin Materials Research Science and Engineering Center (DMR-2309000). Experimental work at LBNL and UC Berkeley was funded by the Quantum Materials (KC2202) program under the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division, under Contract No. DE-AC02-05CH11231 (JO). V.S. and J.O. received support from the Gordon and Betty Moore Foundation’s EPiQS Initiative through Grant GBMF4537 to J.O. at UC Berkeley. O.G. acknowledges support from the U.S. National Science Foundation Graduate Research Fellowship Program for a predoctoral fellowship (grant no. DGE 1752814). D.K.B. also acknowledges support from the Heising–Simons Faculty Fellowship and the Philomathia Foundation. B.H.G. was supported by the Schmidt Science Fellows in partnership with the Rhodes Trust and the Max Planck Society. L.B. and D.A.M. acknowledge support by the NSF Platform for the Accelerated Realization, Analysis, and Discovery of Interface Materials (PARADIM) under cooperative agreement No. DMR-2039380. Electron microscopy was supported by the Platform for the Accelerated Realization, Analysis, and Discovery of Interface Materials (PARADIM) under NSF Cooperative Agreement no. DMR-2039380. This work made use of the Cornell Center for Materials Research (CCMR) Shared Facilities. The FEI Titan Themis 300 was acquired through No. NSF-MRI-1429155, with additional support from Cornell University, the Weill Institute, and the Kavli Institute at Cornell. The Thermo Fisher Helios G4 UX FIB was acquired with support by NSF No. DMR-1539918. The Thermo Fisher Spectra 300 X-CFEG was acquired with support from PARADIM, an NSF MIP (DMR-2039380), and Cornell University. SCXRD and XPS work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.







