Dental enamel—the hardest substance in the human body—is the outer protective layer of teeth. It has evolved to bear large chewing forces, resist mechanical fatigue, and withstand wear over decades. Tooth decay (caries) and loss of dental enamel greatly affect health and quality of life, with associated costs to society.
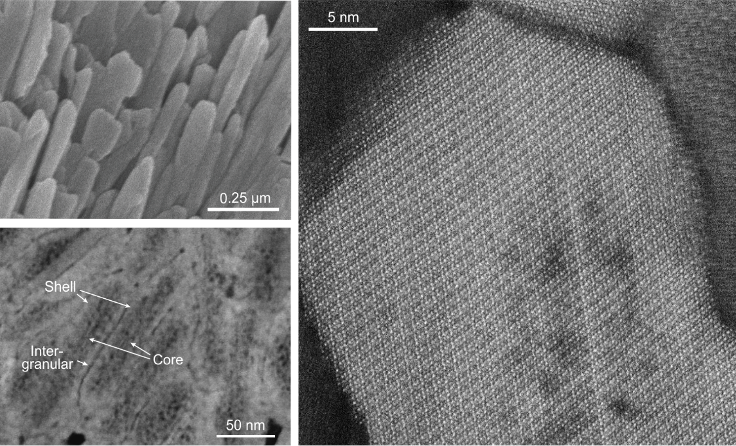
At the nanoscale scale, enamel consists of tightly bunched elongated crystals that are about 1000 times smaller than a human hair. The tiny building blocks of enamel are mostly made of the calcium- and phosphate-based mineral hydroxylapatite.
Now, atomic-scale imaging at PARADIM’s Electron Microscopy facility coupled with atom probe tomography performed at Northwestern University reveals that the uniform arrangement of atoms in the crystallites incorporates further elements (magnesium, sodium, fluorine). Such impurities close to the core of each crystal give rise to strain, which might strengthen the enamel but renders the core more soluble. Exposure to acid led to more erosion in the core compared to the crystallites shell. The new insights will enable a better understanding of enamel degradation, how caries develops, and how to prevent or reverse it.
Karen A. DeRocher, Paul J.M. Smeets et al. Nature 583 (2020) 66–71.
The work has been featured in: Nature Podcast, Cornell Chronicle, Northwestern University, National Institutes of Health, National Institute of Dental and Craniofacial Research, Brinkwire, C&EN, Fast Company, Medical Express, Science Daily, Sci News, and WBBM Radio.
Technical details:
Dental enamel is a principal component of teeth and covers the entire crown of human tooth with a thickness of several millimeters. It has evolved to bear large chewing forces, resist mechanical fatigue, and withstand wear over decades. Functional impairment and loss of dental enamel, caused by developmental defects or tooth decay (caries), affect health and quality of life, with associated costs to society.
Although the past decade has seen progress in our understanding of enamel formation (amelogenesis) and the functional properties of mature enamel, attempts to repair lesions in this material or to synthesize it in vitro have had limited success. This is partly due to the highly hierarchical structure of enamel and additional complexities arising from chemical gradients. Here we show, using atomic-scale quantitative imaging and correlative spectroscopies, that the nanoscale crystallites of hydroxylapatite (Ca5(PO4)3(OH)), which are the fundamental building blocks of enamel, comprise two nanometric layers enriched in magnesium flanking a core rich in sodium, fluoride and carbonate ions; this sandwich core is surrounded by a shell with lower concentration of substitutional defects. A mechanical model based on density functional theory calculations and X-ray diffraction data predicts that residual stresses arise because of the chemical gradients, in agreement with preferential dissolution of the crystallite core in acidic media. Furthermore, stresses may affect the mechanical resilience of enamel. The two additional layers of hierarchy suggest a possible new model for biological control over crystal growth during amelogenesis, and hint at implications for the preservation of biomarkers during tooth development.
This work is part of a PARADIM User Project conceived and performed by researchers of Northwestern University in close collaboration with members of PARADIM’s In-house Research Team located at Cornell University and contributions from the University of Virginia.
Full journal citation: Karen A. DeRocher, Paul J.M. Smeets, Berit H. Goodge, Michael J. Zachman, Prasanna V. Balachandran, Linus Stegbauer, Michael J. Cohen, Lyle M. Gordon, James M. Rondinelli, Lena F. Kourkoutis, and Derk Joester, “Chemical gradients in human enamel crystallites,” Nature 583 (2020) 66–71.https://doi.org/10.1038/s41586-020-2433-3