MIP Highlight #30
Local PARADIM User Project
Metal oxides—also known as ceramics—are commonly used as insulators. Nonetheless, depending on their composition, they do transport electric currents. To model the electronic transport, scientists often rely on a sixty-year-old model, developed for oxides with only one cation type (binary oxides). Unfortunately, this model fails to correctly describe electronic transport for ternary or more complex oxides.
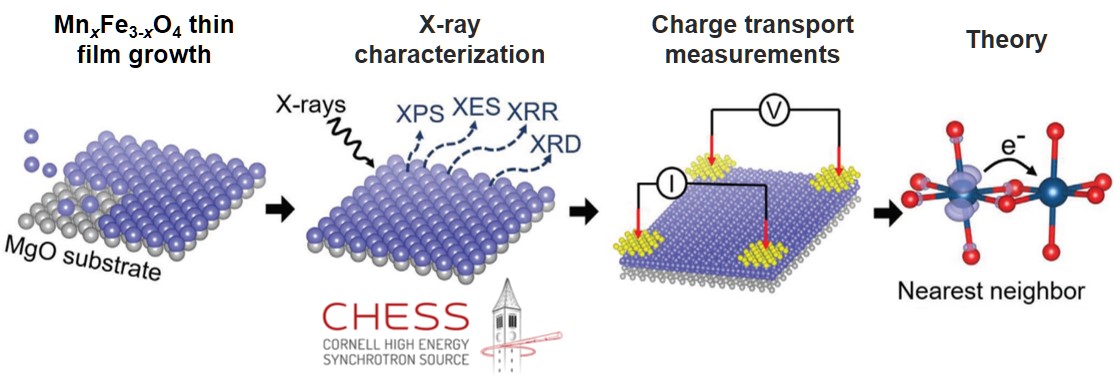
Users of PARADIM put the classic model to a rigorous test. They used PARADIM’s unique synthesis capabilities to prepare single crystals starting from a well-studied binary oxide, Fe3O4. Additional MnxFe3‑xO4 crystals, with higher amounts of Mn incorporated, were prepared without change of the crystal structure. The distribution of atoms within each crystal was determined at PARADIM’s Electron Microscopy facility and CHESS, an NSF-funded research center. By studying the electronic transport in this series of MnxFe3‑xO4 crystals, the classic model was shown to not accurately reproduce the measurements for the ternary oxide.
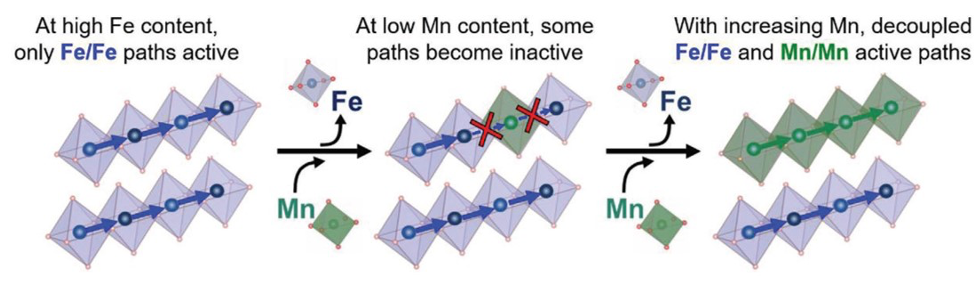
Advanced theoretical simulations and modeling revealed that electricity travels between alike sites—here along distinct Fe or Mn pathways. By revising the classic model to include such transport channels and the ability to jump between transport channels, a new model that accurately describes the transport measurements and is applicable to ternary oxides was born.
What Has Been Achieved: The small-polaron hopping model has been used for six decades to rationalize electronic charge transport in oxides. The model was developed for binary oxides, and, despite its significance, its accuracy has not been rigorously tested for higher-order oxides. Here, the small-polaron transport model is tested by using a spinel system with mixed cation oxidation states (MnxFe3−xO4). Using molecular-beam epitaxy (MBE), a series of single crystal MnxFe3−xO4 thin films with controlled stoichiometry, 0 ≤ x ≤ 2.3, and lattice strain are grown, and the cation site-occupation is determined through X-ray emission spectroscopy (XES). Density functional theory +U analysis shows that charge transport occurs only between like-cations (Fe/Fe or Mn/Mn). The site-occupation data and percolation models show that there are limited stoichiometric ranges for transport along Fe and Mn pathways. Furthermore, due to asymmetric hopping barriers and formation energies, the Mn2+ polaron on charge-conducting octahedral (Oh) sites is energetically preferred to the Oh Fe2+ polaron, resulting in an asymmetric contribution of Mn/Mn pathways. All of these findings are not contained in the conventional small-polaron hopping model, highlighting its inadequacy. To correct the model, new parameters in the nearest-neighbor hopping equation are introduced to account for percolation, cross-hopping, and polaron-distribution, and it is found that a near-perfect correlation can be made between experiment and theory for the electronic conductivity.
Importance of the Achievement: A sixty-year-old model of electrical transport in oxide materials was subjected to a rigorous test. Growing single crystal thin films of the MnxFe3−xO4 model system in which the crystal structure was kept the same and the composition and site occupation was precisely established provided a sample set to systematically study the correlation between elemental composition and electrical transport.
Unique Feature(s) of the MIP that Enabled this Achievement: PARADIM’s unique molecular-beam epitaxy system was used to grow MnxFe3−xO4 single crystals at similar growth conditions over a wide Mn-content range (0 ≤ x ≤ 2.3), despite the large miscibility gap in the bulk phase diagram by exploiting epitaxial stabilization. The structure and site occupancy of the MnxFe3−xO4 single-crystal films was analyzed at PARADIM’s electron microscopy facility and the NSF-funded research center CHESS provided access to advanced X-ray emission spectroscopy.
The work was conceived by Prof. Richard Robinson (Cornell University) a PARADIM user for thin film growth and electron microscopy. Further contributions involve the Cornell NanoScale Science and Technology Facility (CNF), the Cornell High Energy Synchrotron Source (CHESS), the Cornell Center for Materials Research (CCMR), and Technion – Israel Institute of Technology.
Full Reference: A. Bhargava, R. Eppstein, J. Sun, M.A. Smeaton, H. Paik, L.F. Kourkoutis, D.G. Schlom, M. Caspary Toroker, and R.D. Robinson, “Breakdown of the Small-Polaron Hopping Model in Higher-Order Spinels,” Adv. Mater.32, 2004490 (2020).
Data Availability: Experimental data is available at http://data.paradim.org/175/.
The work has been highlighted in the Cornell Chronicle: https://news.cornell.edu/stories/2020/10/collaboration-sparks-new-model-ceramic-conductivity.
Acknowledgement: This work was supported in part by the National Science Foundation (NSF) under award number DMR-1809429 and CHE-1507753. This work made use of the Platform for the Accelerated Realization, Analysis, and Discovery of Interface Materials (PARADIM), Cornell Center for Materials Research (CCMR), and Cornell High Energy Synchrotron Source (CHESS) facilities and was funded in part by the National Science Foundation under award numbers DMR-1539918, DMR-1719875, and DMR-1829070, respectively. M.A.S. acknowledges support from the NSF GRFP under award number DGE-1650441. The FEI Titan Themis 300 was acquired through grant no. NSF-MRI-1429155, with additional support from Cornell University, the Weill Institute, and the Kavli Institute at Cornell. Substrate preparation was performed in part at the Cornell NanoScale Facility, a member of the National Nanotechnology Coordinated Infrastructure (NNCI), which is supported by the NSF (grant no. ECCS-1542081). This research was also supported by a grant from the United States-Israel Binational Science Foundation (BSF), Jerusalem, Israel, and the United States National Science Foundation (NSF). This research was supported by the Nancy and Stephen Grand Technion Energy Program (GTEP). This article is based upon the work from COST Action 18234, supported by COST (European Cooperation in Science and Technology). The authors also thank Kenneth D. Finkelstein and Christopher J. Pollock for helping with the XES experiments, and Muhammad Salim for helping with the XPS experiments.
The work has been highlighted:
- in the Cornell Chronicle: https://news.cornell.edu/stories/2020/10/collaboration-sparks-new-model-ceramic-conductivity
- Selected as Frontispiece, December (2020) Advanced Materials.