High entropy compounds are an emerging class of functional materials in which short range order enables superior combinations of properties not present in traditional pure or doped structures.
 |
|
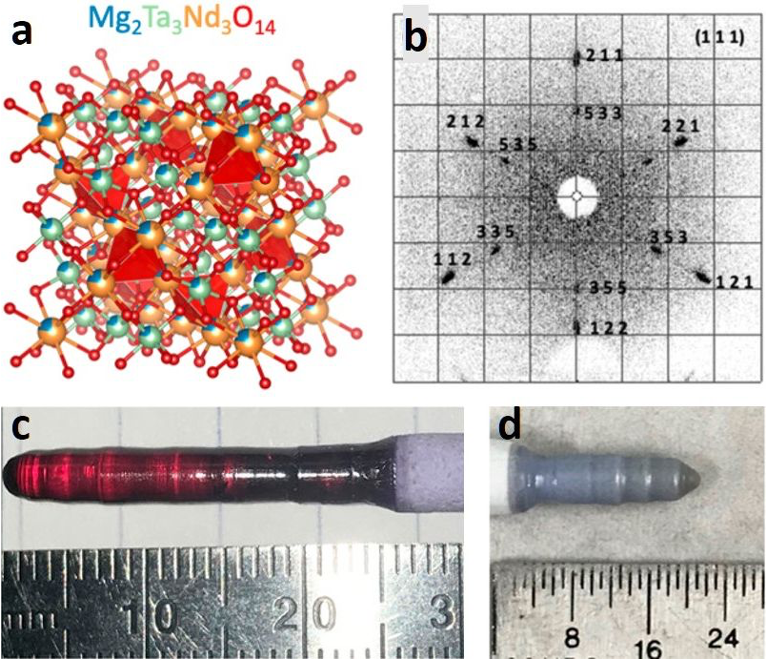
Figure 1: a) Model of crystal structure of Mg2Ta3Nd3O14. b) Laue image showing single crystalline nature of the grown Boule. c) Single crystal of Mg2Ta3Nd3O14 grown using PARADIM’s Laser Diode Floating Zone. The red color is due to oxygen deficiencies that upon annealing in air turn back to the expected blue of Nd compounds. d) Single crystal of Mg2Ta3Nd3O14 grown using PARADIM’s Laser Diode Floating Zone. |
Members of the PARADIM in-house research team discovered and synthesized a family of high entropy oxides with the formula Mg2Ta3Ln3O14 (Ln = La, Pr, Nd, Sm, Eu, Gd) using the laser optical floating zone technique. This family of materials can host a variety of cation defects and oxygen vacancies that give it a “dial-in” lattice parameter, suggesting applications as a tunable substrate for thin film growth. The crystals’ oxygen concentration has direct impact on the optical properties. The ability to visually observe color changes at high temperatures demonstrates the possibility of using this as an active element in optical-based oxygen sensors.
Centimeter-sized single crystals for Ln = Nd have been grown—one of the few demonstrated high entropy materials—showing the capabilities of the Laser Diode Floating Zone available to users of PARADIM.
What Has Been Achieved: A family of high entropy oxides with the formula Mg2Ta3Ln3O14 (Ln = La, Pr, Nd, Sm, Eu, Gd) has been discovered and synthesized. Centimeter-sized single crystals for Ln = Nd have been grown using the laser optical floating zone technique, one of the first successful growths of a high entropy material.
Importance of the Achievement: High entropy compounds are an emerging class of functional materials in which short range order enables superior combinations of properties not present in traditional pure or doped structures. However, this structural complexity also makes production of single crystals – needed for fundamental studies and some of the most compelling applications – difficult due to the presence of multiple competing phases. This work demonstrates that such single crystals are indeed possible. Further, this family can host a variety of cation defects and oxygen vacancies that give it a “dial-in” lattice parameter, suggesting applications as a tunable substrate for thin film growth. The ability to visually observe color changes in the crystal’s oxygen concentration at high temperatures demonstrates the possibility of using this as an active element in optical-based oxygen sensors. This work is also unusual in having a high school student as a co-author on the work, helping spread the importance of STEM and materials to younger ages.
Unique Feature(s) of the MIP that Enabled this Achievement: This is one of the few demonstrated single crystals of high entropy materials, showing the capabilities of the Laser Diode Floating Zone at PARADIM to grow new materials.
Members of PARADIM’s In-House Research team conceived and performed the work.
Publication: L.A. Pressley, A. Torrejon, W.A. Phelan, and T.M. McQueen, "Discovery and Single Crystal Growth of High Entropy Pyrochlores," Inorg. Chem. 59, 17251-17258 (2020).
Data Availability: Raw data is made available at https://data.paradim.org/99002 and the crystal structure datafile at https://doi.org/10.25505/fiz.icsd.cc25y4dp.
Acknowledgement: This work was funded by the Platform for the Accelerated Realization, Analysis, and Discovery of Interface Materials (PARADIM), a National Science Foundation Materials Innovation Platform (NSF DMR-1539918). Angela Torrejon gratefully acknowledges that research was sponsored by the Army Educational Outreach Program (AEOP) and was accomplished under AEOP Research & Engineering Apprenticeship Program FY20 Site Agreement. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Educational Outreach Program or the U.S. Government. The U.S. Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation herein. L.A.P. would like to thank Dr. Maxime Siegler for helping with single crystal data collection, Veronica Stewart with assistance in PPMS measurements, and Juan Chamarro, Hector Vivanco, Tanya Berry, and Mekhola Sinha for their helpful conversations relating to heat capacity analysis.







