PARADIM Highlight #91—In-House Research (2024)
L.F. Kourkoutis, D.G. Schlom, and K.M. Shen (Cornell University)
Nickelates have been the subject of considerable interest because they are close cousins of the well-known "cuprates,” a family of copper oxide-based superconductors that can have high transition temperatures, upwards of 100 Kelvin, at which point electrical resistance vanishes. Superconducting nickelate analogs of the cuprates have long been pursued and finally achieved in thin films using a rather involved processes.
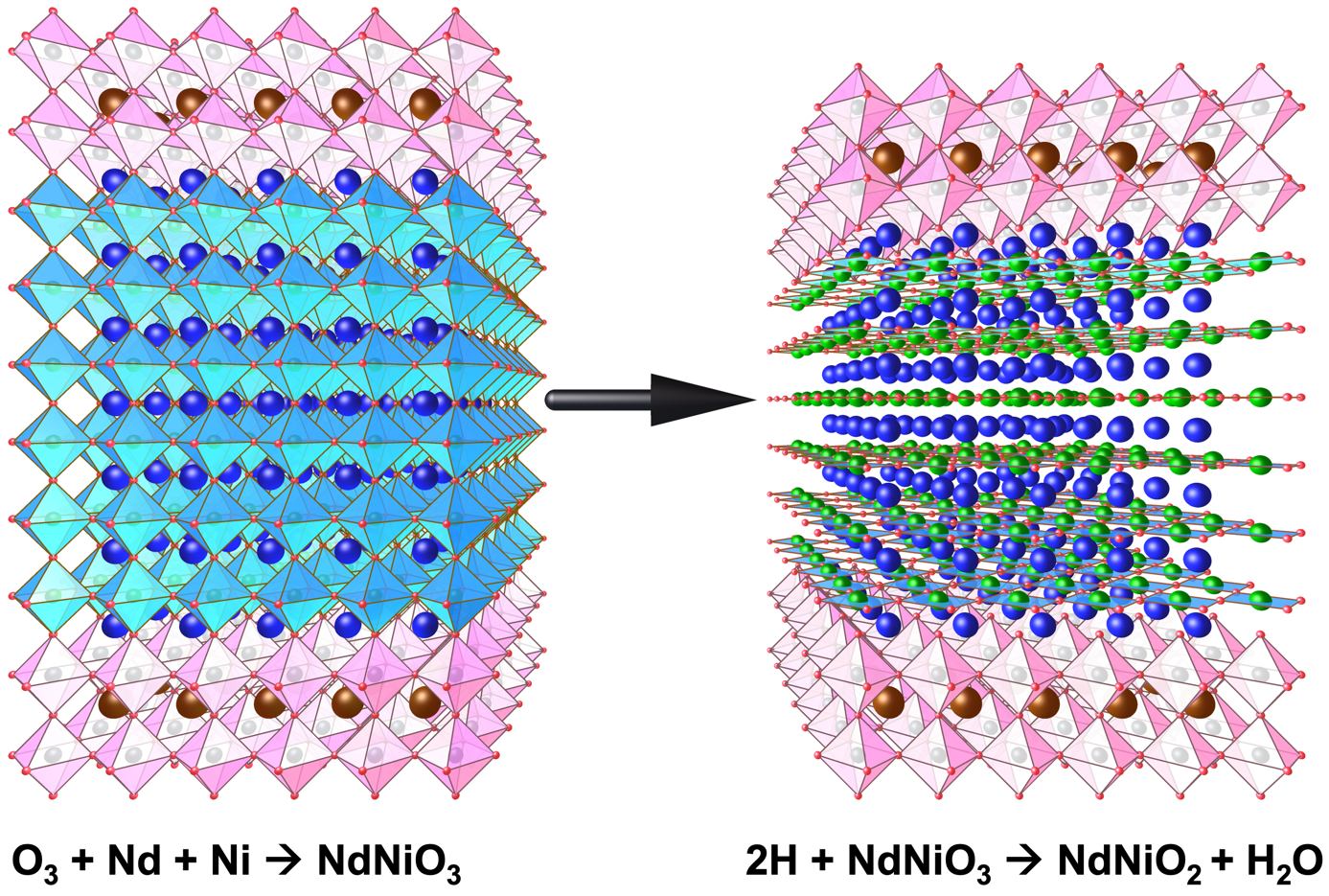
Figure 1: Schematic of the two-step in situ synthesis of NdNiO2. (left) First the NdNiO3 film (shown in blue) is grown by MBE. (right) Second, the temperature is lowered, and atomic hydrogen is used to scavenge oxygen from the film and reduce it to NdNiO2. A capping layer of SrTiO3 (shown in pink) helps to avoid damage to the surface of the NdNiO2 layer and promotes uniform oxygen removal.
Here, members of PARADIM’s In-House Research Team described a novel, flexible synthesis route for infinite layer nickelate films—one that requires only gas phase reactants and is easily integrable with thin film growth techniques and surface sensitive probes. The gentle reduction of NdNiO3 to NdNiO2 in a way that preserves its crystal structure makes use of atomic hydrogen and can be accomplished in short periods (<20 minutes). Structural and electrical transport measurements of the resulting films show high crystallinity, fewer defects, low resistivity, and flat surfaces. Utilizing the addition of an ultrathin protection layer of SrTiO3 just 1-3 atomic layers thick (0.4-1.2 nm), drastically improves the reduction process and inhibits the formation of a polycrystalline scale layer on the sample surface.
PARADIM’s In-House Team presents an integrated procedure for the synthesis of infinite-layer nickelates using molecular-beam epitaxy with gas-phase reduction by atomic hydrogen. Already the growth of perovskite NdNiO3/SrTiO3 is challenging, arising from post growth crack formation in stoichiometric films. The procedure for fully reducing NdNiO3 films to the infinite-layer phase, NdNiO2, uses atomic hydrogen; the resulting films display excellent structural quality, smooth surfaces, and lower residual resistivities than films reduced by other methods. Thanks to the in situ nature of this technique the impact of an ultra-thin SrTiO3 capping layers on the reduction process can be shown and highlights their importance in preventing the formation of secondary phases at the exposed nickelate surface. A comparative bulk- and surface-sensitive study indicates formation of a polycrystalline crust on the film surface serves to limit the reduction process. Ref. 1.
A hallmark of many unconventional superconductors is the presence of many-body interactions that give rise to broken-symmetry states intertwined with superconductivity. Recent resonant soft X-ray scattering experiments report commensurate 3a0 charge density wave order in infinite-layer nickelates, which has important implications regarding the universal interplay between charge order and superconductivity in both cuprates and nickelates. Here, PARADIM presents X-ray scattering and spectroscopy measurements on a series of NdNiO2+x samples, which reveal that the signatures of charge density wave order are absent in fully reduced, single-phase NdNiO2. The 3a0 superlattice peak instead originates from a partially reduced impurity phase where excess apical oxygens form ordered rows with three-unit-cell periodicity. The absence of any observable charge density wave order in NdNiO2 highlights a crucial difference between the phase diagrams of cuprate and nickelate superconductors, see Ref 2.
This achievement was made possible by the electron microscopy and thin film growth facilities of PARADIM. It is the first work to utilize atomic hydrogen to accomplish the topotactic reduction of NdNiO3, with the perovskite structure, to NdNiO2, with the “infinite-layer” structure. Subsequent work by other groups has used atomic hydrogen to reduce La0.8Sr0.2NiO3 to superconducting La0.8Sr0.2NiO2 and also to reveal ARPES on such reduced films (see arXiv:2401.15979 and arXiv:2403.07344).
- C.T. Parzyck, V. Anil, Y. Wu, B.H. Goodge, M. Roddy, L.F. Kourkoutis, D.G. Schlom, and K.M. Shen, “Synthesis of Thin Film Infinite-Layer Nickelates by Atomic Hydrogen Reduction: Clarifying the Role of the Capping Layer,” APL Materials 12, 031132 (2024).
- C.T. Parzyck, N.K. Gupta, Y. Wu, V. Anil, L. Bhatt, M. Bouliane, R. Gong, B.Z. Gregory, A. Luo, R. Sutarto, F. He, Y.-D. Chuang, T. Zhou, G. Herranz, L.F. Kourkoutis, A. Singer, D.G. Schlom, D.G. Hawthorn, and K.M. Shen, “Absence of 3a0 Charge Density Wave Order in the Infinite-Layer Nickelates NdNiO2,” Nature Materials 23, 486–491 (2024).
- This work was primarily supported by the National Science Foundation through Grants No. DMR-2104427 and the Platform for the Accelerated Realization, Analysis and Discovery of Interface Materials (PARADIM) under Cooperative Agreement No. DMR-2039380. Additional support was provided by the Air Force Office of Scientific Research (Grant No. FA9550-21-1-0168) and the Gordon and Betty Moore Foundation’s EPiQS Initiative through Grant Nos. GBMF3850 and GBMF9073.. The authors acknowledge the use of facilities and instrumentation supported by NSF through the Cornell University Materials Research Science and Engineering Center DMR-1719875. The FEI Titan Themis 300 was acquired through No. NSF-MRI-1429155, with additional support from Cornell University, the Weill Institute, and the Kavli Institute at Cornell. The Thermo Fisher Helios G4 UX FIB was acquired with support by NSF No. DMR-1539918. Substrate preparation was performed, in part, at the Cornell NanoScale Facility, a member of the National Nanotechnology Coordinated Infrastructure (NNCI), which is supported by the National Science Foundation (Grant NNCI-2025233). The authors would like to thank Sean Palmer and Steven Button for their assistance in substrate preparation as well as Hari Nair and Jiaxin Sun for their thoughtful discussions and insightful Comments.
- In memory of our colleague Lena F. Kourkoutis, an outstanding scientist, mentor and microscopist. This work was primarily supported by the US Department of Energy (DOE), Office of Basic Energy Sciences, under contract no. DE-SC0019414. This research used resources of the Advanced Light Source, a US DOE Office of Science User Facility, under contract no. DE-AC02-05CH11231, as well as the Center for Nanoscale Materials and the Advanced Photon Source, both US DOE Office of Science User Facilities operated for the DOE Office of Basic Energy Sciences by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Part of the research described in this paper was performed at the Canadian Light Source, a national research facility of the University of Saskatchewan, which is supported by the Canada Foundation for Innovation (CFI), the Natural Sciences and Engineering Research Council (NSERC), the National Research Council (NRC), the Canadian Institutes of Health Research (CIHR), the Government of Saskatchewan and the University of Saskatchewan. Additional support for materials synthesis was provided by the Air Force Office of Scientific Research (grant no. FA9550-21-1-0168), the National Science Foundation (no. DMR-2104427) and the Gordon and Betty Moore Foundation’s EPiQS Initiative through grant nos. GBMF3850 and GBMF9073. Substrate preparation was performed in part at the Cornell NanoScale Facility, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the NSF (grant no. NNCI-2025233). We would like to thank S. Palmer and S. Button for their assistance in substrate preparation. STEM characterizations were performed at the Cornell Center for Materials Research Facilities supported by the National Science Foundation (DMR-1719875). The microscopy work at Cornell was supported by the NSF PARADIM (DMR-2039380), with additional support from Cornell University, the Weill Institute and the Kavli Institute at Cornell. L.B. and L.F.K. acknowledge support from the Packard Foundation. G.H. acknowledges support from Severo Ochoa FUNFUTURE (no. CEX2019-000917-S) of the Spanish Ministry of Science and Innovation and by the Generalitat de Catalunya (2021 SGR 00445). K.M.S. would like to acknowledge the hospitality of ICMAB-CSIC during his sabbatical. We would like to sincerely thank J. Fontcuberta, H. Y. Hwang, W. S. Lee and D. A. Muller for helpful discussions and insights.
Data Availability: Data associated with the growth of the samples in PARADIM’s Thin Film Growth facility is available through the PARADIM Data Collective (PDC) at DOI: 10.34863/44w9-wc96.







