PARADIM Highlight #107—External User Project (2025)
Kwabena Bediako (UC Berkeley)
Low-dimensional materials provide a framework for nanoscale functional devices with properties that can be flexibly designed for a wide range of applications, from next-generation computing to data storage and more. Stacking multiple materials into layered vertical heterostructures has been a particularly successful strategy for combining multiple emergent physical properties such as magnetism or superconductivity in a single bespoke architecture. Traditional synthesis approaches for such heterostructures, however, are often limited to materials with specific structural requirements, such as those with similar crystalline lattices or those which can be easily exfoliated into stackable sheets, which can limit either the flexibility or performance of resulting devices.
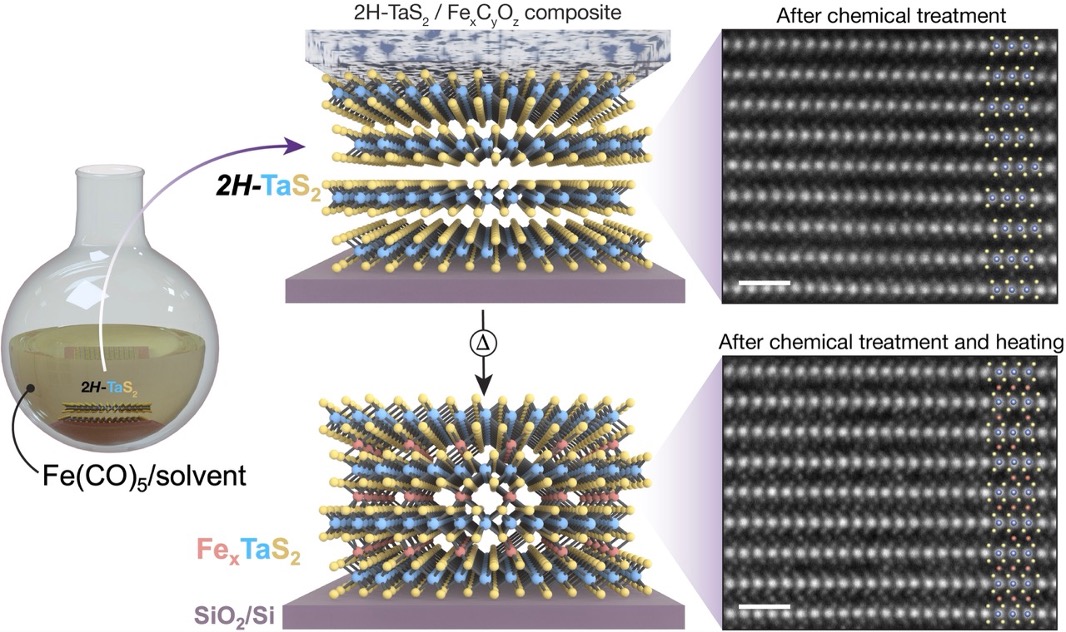
Figure 1:(Left) Schematic of depositing Fe precursors onto 2H–TaS2 crystals on SiO2/Si support followed by vacuum annealing to yield Fe-intercalated 2H–TaS2 (FexTaS2). (Right) Atomic resolution electron microscopy after chemical treatment (top) plus annealing at 350 °C for 30 minutes (bottom). Scale bars 1 nm.
Here, a group from UC Berkeley demonstrates a new method for synthesizing heterostructures of magnetic intercalation compounds of transition metal dichalcogenides (TMDs) through directed chemical reactions. Leveraging PARADIM’s capabilities for atomic-resolution imaging and spectroscopy of sensitive materials, we show that the chemical intercalation of magnetic ions is a thermally driven—contrary to previous belief. Our improved understanding of the intercalation chemistry enables us to design and realize more complex intercalated heterostructures, with independent control over both the host material and intercalant species within constituent layers. This advance opens the door to new materials combinations for both fundamental research and functional devices.
This work establishes a new route to highly customizable construction of electronic or magnetic devices. Investigating multiple stages of the device fabrication process with atomic-resolution imaging and spectroscopy, we show that thermal annealing drives chemical intercalation of magnetic ions in precursor films patterned on TMD flakes. We reveal key details of the intercalation process, for example, lateral diffusion of magnetic ions along the atomic planes. Leveraging these insights, we use controlled patterning to direct metal ions into well-defined van der Waals (vdW) heterostructures, expanding the range of accessible low-dimensional magnetic intercalation compounds as well as producing magnetic multilayer systems with exceptionally clean surfaces and interfaces. We demonstrate the flexibility and promise of our approach through proof-of-concept devices and experiments, including nano-ARPES measurements of pristine exposed surfaces, hybrid heterostructures with mixed intercalants (Fe and Co) or TMD layers (NbS2 and TaS2), and measurements suggesting strong coupling across atomically pristine interfaces between them.
Intercalated heterostructures are compelling platforms for multi-component spintronic device architectures, including spin-orbit torque devices, magnetic tunnel junctions, and memristors. Established techniques such as mechanical exfoliation and assembly for fabricating heterostructures of layered crystals, however, are not viable for intercalated crystals: the strong interlayer interactions imparted by the intercalants pose significant challenges for isolating the thin crystals which are integral components of heterostructures. By comparison, our approach combines microscale patterning techniques with solid-state chemical reactions to create pristine heterostructures of magnetic intercalation compounds with tunable dimensionality, atomically sharp interfaces, and ultraclean surfaces. Furthermore, this work suggests that the emergent physics of these heterostructures may be controlled by altering the thickness of constituent flakes and the intercalants’ identity, stoichiometry, and homogeneity. Additionally, we expect that the synthesis of heterostructures with atomically sharp moiré heterointerfaces will offer exciting opportunities to both control the intercalation chemistry and also to fundamentally engineer complex magnetic behaviors. This customizability of vdW heteroepitaxy combined with nanoscale intercalation chemistry unlocks versatile opportunities to fine-tune magnetic behaviors in functional materials.
This work was made possible by the low-dose imaging and spectroscopy in PARADIM’s high-brightness Spectra “Kraken” STEM required to achieve atomic resolution visualization and elemental mapping of the sensitive Fe intercalants which are rapidly damaged under more conventional experimental conditions.
S. Husremović, O. Gonzalez, B.H. Goodge, L.S. Xie, Z. Kong, W. Zhang, S.H. Ryu, S.M. Ribet, S.S. Fender, K.C. Bustillo, C. Song, J. Ciston, T. Taniguchi, K. Watanabe, C. Ophus, C. Jozwiak, A. Bostwick, E. Rotenberg, and D.K. Bediako, "Tailored Topotactic Chemistry Unlocks Heterostructures of Magnetic Intercalation Compounds," Nat. Commun. 16, 1208 (2025). DOI: 10.1038/s41467-025-56467-9
We thank R. Murphy and M.P. Erodici for helpful discussions, and we acknowledge C. Gammer and S. Zeltmann for developing the 4D-STEM acquisition code for TitanX. We also acknowledge G.P. Hegel for help with bulk characterization. Research supported by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES), under Award # DE-SC0025525. This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. Work at the Molecular Foundry, LBNL, was supported by the Office of Science, Office of Basic Energy Sciences, the U.S. Department of Energy under Contract no. DE-AC02-05CH11231. Confocal Raman spectroscopy was supported by a Defense University Research Instrumentation Program grant through the Office of Naval Research under award no. N00014-20-1-2599 (D.K.B.). Portions of this work were supported by the Gordon and Betty Moore Foundation EPiQS Initiative Award no. 10637 and the Heising Simons Faculty Fellowship. Electron microscopy was, in part, supported by the Platform for the Accelerated Realization, Analysis, and Discovery of Interface Materials (PARADIM) under NSF Cooperative Agreement no. DMR-2039380. This work made use of the Cornell Center for Materials Research (CCMR) Shared Facilities, which are supported through the NSF MRSEC Program (no. DMR- 1719875). The Thermo Fisher Spectra 300 X-CFEG was acquired with support from PARADIM, an NSF MIP (DMR-2039380) and Cornell University. K.W. and T.T. acknowledge support from JSPS KAKENHI (Grant Numbers 19H05790, 20H00354 and 21H05233). B.H.G. was supported by the University of California Presidential Postdoctoral Fellowship (PPFP) and the Schmidt Science Fellows, in partnership with the Rhodes Trust. L.S.X. was supported by an Arnold O. Beckman Postdoctoral Fellowship. S.H. acknowledges support from the Blavatnik Innovation Fellowship.
Data Access: DOI 10.5281/zenodo.13821437







